Kidney Tutorial
Gilead Confidential & Proprietary—Do Not Copy or Distribute. This information is provided for background information only. All product discussions must be consistent with the prescribing information and fairly balanced.
The purpose of this tutorial is to provide you with a working knowledge of how the kidneys work, how renal function is measured, the basics of acute and chronic kidney disease (CKD), how the hepatitis B virus (HBV) can affect the kidneys, how oral antiviral agents can affect the kidneys, and potential dosing considerations for patients with renal impairment. This module will help you identify where tenofovir alafenamide (TAF) comes into play when choosing a clinically appropriate oral antiviral for the treatment of chronic hepatitis B (CHB).
Lesson 1. The Kidneys in Health

Patient Perspective: Alan
In this training module we will follow the story of Alan, a 57-year-old second-generation Asian American who has been diagnosed with CHB. As you'll recall, Alan's physician, Dr Thompson, has determined that he is a candidate for antiviral therapy.
A glance at Alan's chart shows that he has a BMI of 30 and a history of hypertension and dyslipidemia. Before initiating antiviral therapy, Dr Thompson explains that it's important to evaluate his overall health, including how well his kidneys are functioning. Alan admits to Dr Thompson that he really doesn't know that much about the kidneys, or why they are important.
In this lesson, we'll start by learning how the kidneys normally function in health.
Lesson Introduction
This lesson describes the structure and function of the kidney, with an emphasis on the filtering units of the kidney, the nephron, and the process by which blood is filtered and urine is formed. This information will lay the foundation for understanding how kidney function is measured and understanding what goes wrong in kidney disease.
Learning Objectives
A Closer Look at Hormones Produced by the Kidneys
Three key hormones are produced by the kidneys:
- Calcitriol helps regulate calcium homeostasis
- Erythropoietin stimulates the production of red blood cells
- Renin helps to regulate blood pressure
After completing this lesson, you will be able to:
- Briefly describe kidney functions
- Identify the key structures of the nephron
- Describe the 3 major steps in blood purification within the kidney
Physiologic Functions of the Kidney
The kidneys do the major work of the urinary system and participate in many physiologic processes essential to life, including:
- Excretion of wastes and foreign substances through the formation of urine
- Regulation of the composition of the blood, including electrolyte balance, blood pH, and blood glucose, and the volume of blood, which contributes to regulation of blood pressure
- Production of the hormones calcitriol, erythropoietin, and renin
The kidneys filter nearly 200 liters of blood daily. This is equivalent to filtering the entire volume of plasma in the body more than 60 times each day.
Why Is It Important for You to Understand Renal Physiology?
Learning key concepts and terms related to renal physiology will prepare you to understand how HBV infection and certain medications used in its treatment can affect the kidneys, and about the clinical considerations associated with managing HBV in patients with chronic kidney disease (CKD).
Anatomy of the Kidneys
The kidneys are paired, bean-shaped organs that lie immediately above the waist, behind the peritoneum of the abdominal cavity. (See Figure 1.)
Figure 1. Anatomy of the Kidney
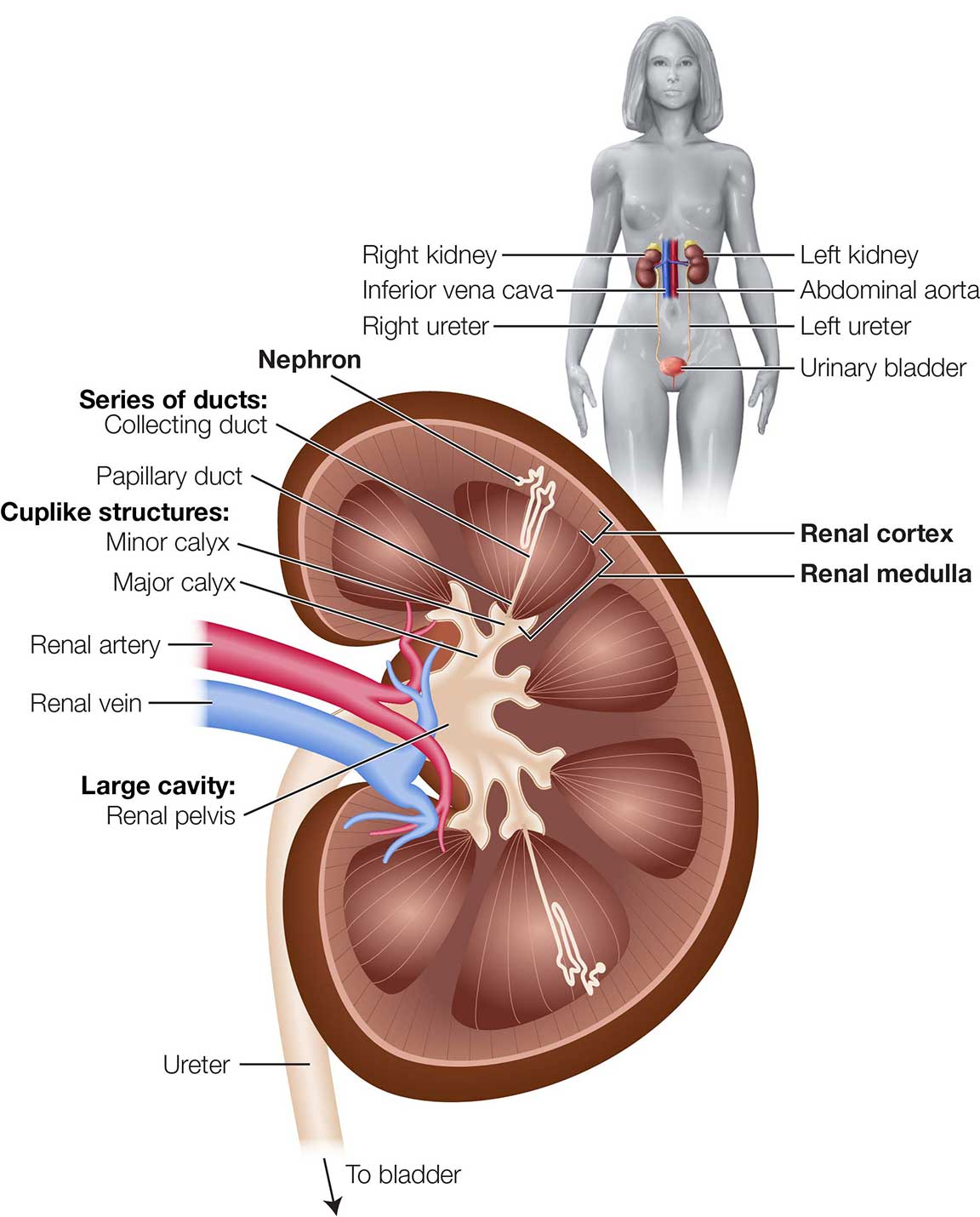
Each kidney has 3 distinct regions:
- Renal cortex—the most superficial region
- Renal medulla—lies beneath the renal cortex and consists of cone-shaped renal pyramids
- Renal pelvis—funnel-shaped area in the center of each kidney where urine collects
Together, the renal cortex and renal pyramids of the medulla comprise the functional portion, or parenchyma, of the kidney. The main functional units of the kidneys, the nephrons, are located within the parenchyma. Each kidney has approximately 1 million nephrons.
Extensions of the renal pelvis called calyces (singular: calyx) surround the renal pyramids and collect urine formed by the kidneys, ultimately draining into the ureter, a tube-like structure that carries urine to the urinary bladder so that it can be stored and then eliminated.
Blood Flow Through the Kidneys
To fulfill their purpose of filtering blood and removing wastes, the kidneys require a rich blood supply. Each kidney is supplied by a renal artery, which carries blood into the kidney. Under normal resting conditions, the renal arteries deliver one-fourth of the cardiac output (the total volume of blood pumped out by the heart each minute) to the kidneys.
Within each kidney, the renal artery branches into increasingly smaller arteries that carry blood to different areas of the kidney. Ultimately, these vessels branch into afferent arterioles, which carry blood into each nephron, as described below.
Blood leaving the renal cortex drains into a series of veins that ultimately merge to form the renal vein, which carries blood out of the kidney so that it can be returned to the heart.
A Closer Look at the Nephron
Nephrons are the main functional units of the kidney, where blood is processed and urine is formed. Because these nephrons are so important to your understanding of renal function, let's take a closer look at them.
The structure of a nephron is shown in Figure 2.
Figure 2. The Nephron
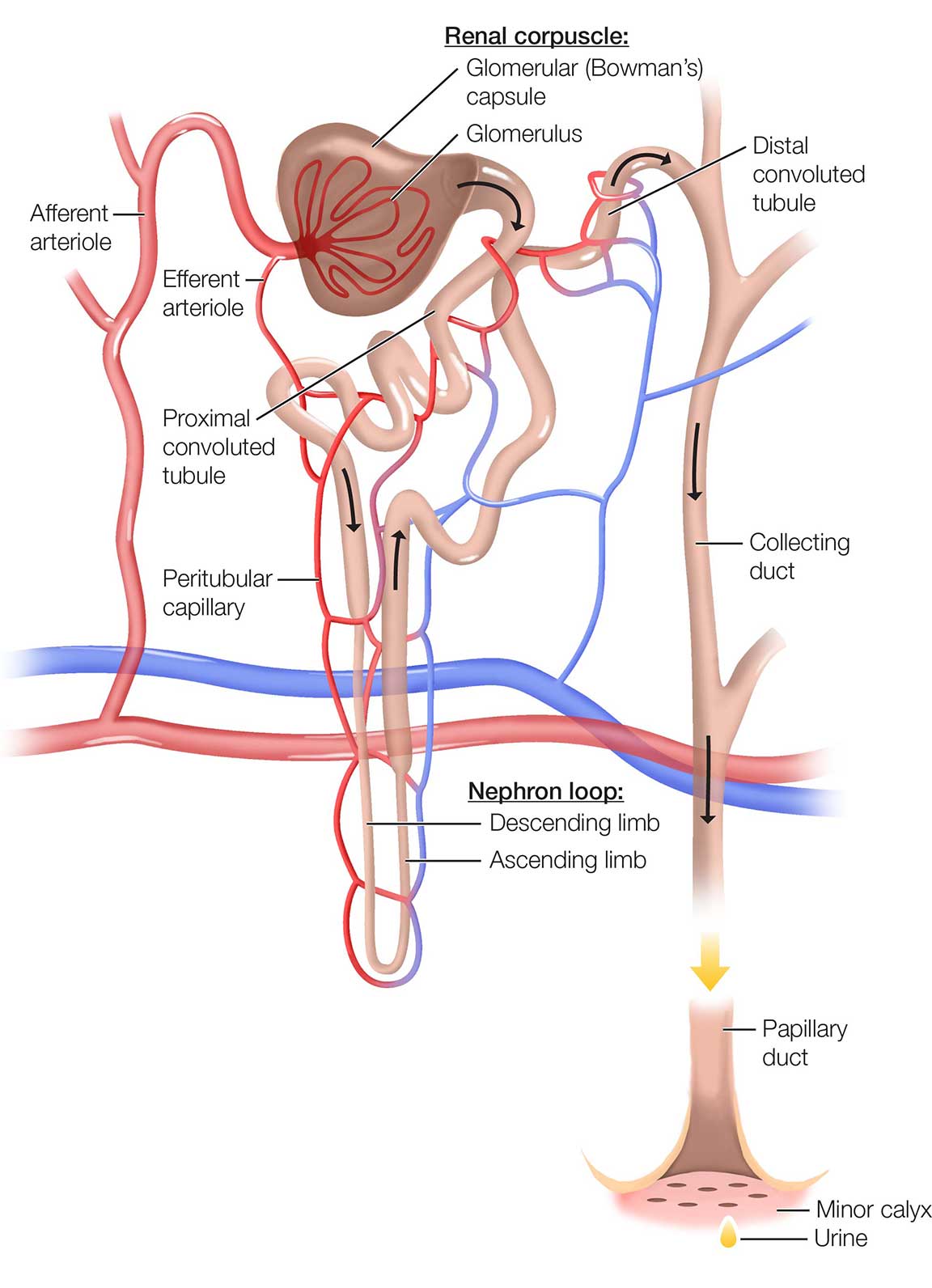
The 2 major parts of the nephron are the renal corpuscle and the renal tubules. The renal corpuscles are located in the renal cortex, and the renal tubules begin in the cortex and pass into the medulla before returning to the cortex. These structures are intimately connected; as blood flows through the nephron to be filtered, water and other substances move between the renal corpuscle and the tubules.
Renal corpuscle
The renal corpuscle consists of a glomerulus, a dense network of capillaries and porous membrane that selectively allows substances to pass through it, and a Bowman's capsule (also known as glomerular capsule), which collects the glomerular filtrate and passes it to the tubule.
The glomerular capillaries are formed by branches of the afferent arteriole supplying the nephron. The glomerular capillaries reunite to form the efferent arteriole, which carries blood out of the glomerulus. The efferent arteriole then branches into the peritubular capillaries, which surround the renal tubules.
Renal tubule
The renal tubule has 3 major subdivisions:
- Proximal convoluted tubule—twisted, winding region immediately after the Bowman's capsule
- Loop of Henle (sometimes referred to as the nephron loop)—long, hairpin-shaped loop after the proximal tubule
- Distal convoluted tubule—a long tubule that is distal to the loop of Henle
The distal convoluted tubules from multiple nephrons empty into a single collecting duct. Collecting ducts collect the fluid processed by nephrons and convey it to the renal pelvis.
Urine Formation
The function of the nephrons is to eliminate waste substances from the blood so that they can be excreted via the urine, while reabsorbing essential substances back into the blood. In doing so, nephrons help maintain homeostasis of the blood's volume and composition.
Urine formation is illustrated in Figure 3.
Figure 3. Urine Formation
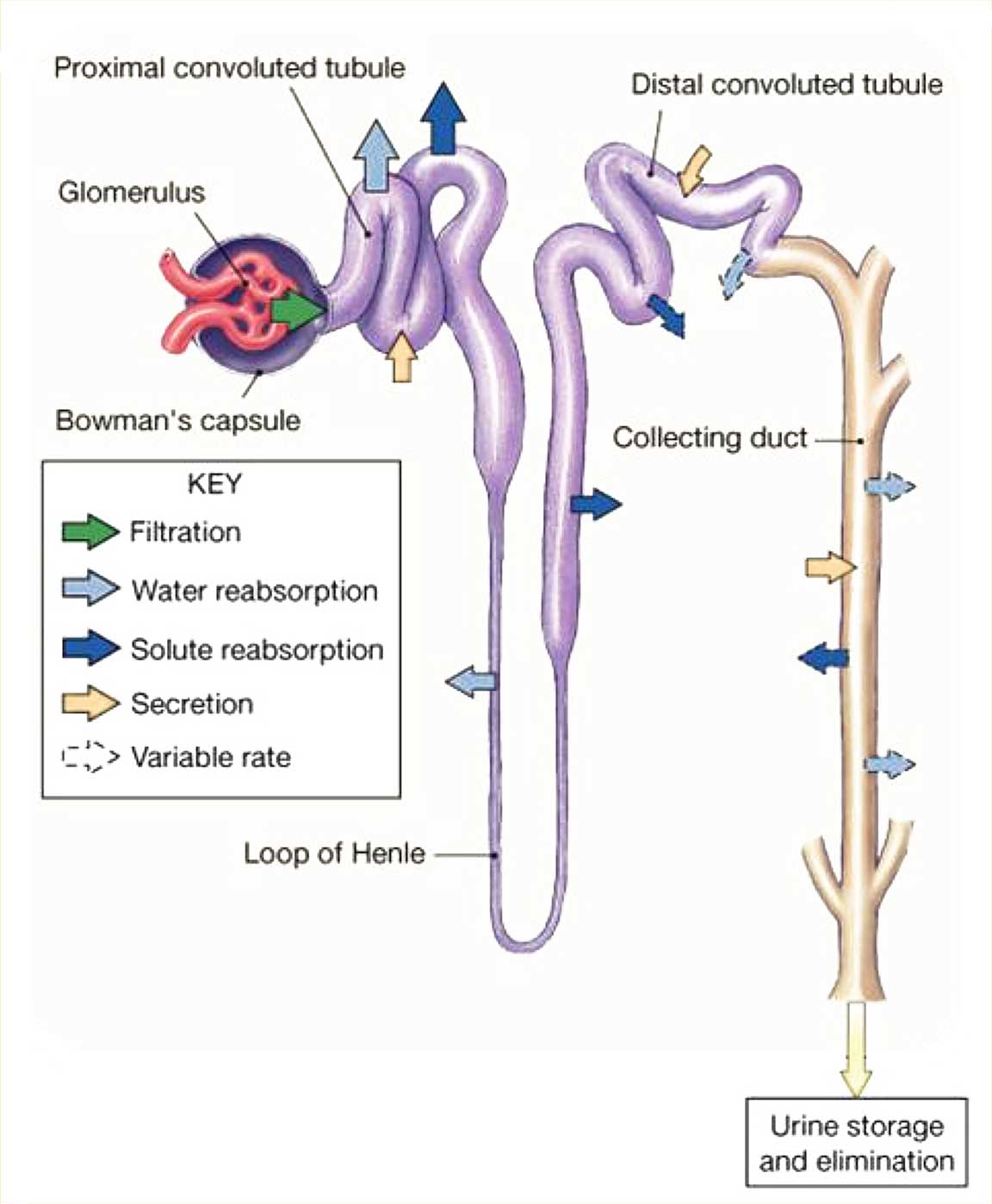
There are 3 main aspects to the production of urine by the kidneys, with most of the key steps occurring in the nephron.
- Glomerular filtration. This first step in the formation of urine occurs in the renal corpuscle; here, blood plasma and substances dissolved in the blood get filtered into the glomerular capsule. This step produces a filtrate that is free of cells and proteins.
- Tubular reabsorption. This is the process of selectively moving substances from the filtrate back into the blood. As filtrate flows through the renal tubules and through the collecting ducts, cells in the tubules reabsorb about 99% of the filtered water and many useful solutes (eg, glucose, amino acids) so that they can be returned to the blood.
- Tubular secretion. This is the process of selectively moving substances from the blood into filtrate. As filtered fluid moves through the renal tubules, cells there secrete other materials, such as wastes, drugs, and excess ions, into the renal tubule.
Ultimately, filtrate drains from the nephrons into the papillary ducts, which extend through the renal pyramids before draining into the minor and major calyces. The filtrate eventually drains into the ureter so that it can be stored in the urinary bladder and then excreted.
Clinical Connection: Glomerular Filtration Rate
Glomerular filtration rate (GFR) is the amount of filtrate formed in all renal corpuscles of both kidneys each minute. In order to maintain homeostasis of body fluids, the GFR must remain relatively constant.
- If the GFR is too high, needed substances may pass so quickly through the renal tubules that some are not reabsorbed and are lost through excretion in the urine.
- If the GFR is too low, nearly all of the filtrate may be reabsorbed and certain waste products may not be adequately excreted.
The role of GFR in the evaluation of kidney function and how it is measured is discussed in Lesson 2.

Patient Perspective: Alan
Dr Thompson explains to Alan that the kidneys do more than produce urine; they filter the blood, help maintain normal electrolyte balance, secrete essential hormones, and even help regulate blood pressure.
Lesson Summary
- The kidneys participate in many key physiologic processes, including excretion of wastes and foreign substances through the formation of urine, regulation of the composition and volume of blood, and production of the hormones calcitriol, erythropoietin, and renin.
- The kidneys reside within the abdominal cavity. The outer layer of the kidney is the renal cortex and the inner layer is the renal medulla. The renal artery carries blood into the kidney and renal veins carry blood from the kidneys.
- The nephron—the main functional unit of the kidney—is where blood is processed and urine is formed. The nephron's 2 main components are the renal corpuscle and the renal tubules, and both participate in blood processing and urine formation.
- The following are the 3 main steps in urine production:
- Glomerular filtration: Blood plasma and substances dissolved in the blood get filtered into the glomerular capsule to produce a filtrate that is free of cells and proteins.
- Tubular reabsorption: Substances selectively move from the filtrate back into the blood, reabsorbing most of the water and many useful solutes.
- Tubular secretion: Substances selectively move from the blood into the filtrate, and wastes, drugs, excess ions, and other materials are secreted into the renal tubule.
- From the renal tubule, the urine that is formed flows into the collecting duct and ultimately leaves the kidney via the ureter so that it can be excreted.
Self-Check Questions
1. Name 3 functions of the kidneys.
Take a moment to think about the answer to this question, and then select the button to check your answer.
Correct Answer
Compare your answer to the correct answer below:
- Excretion of wastes and foreign substances via urine formation
- Regulation of the composition and volume of blood
- Production of hormones, such as calcitriol, erythropoietin, and renin
Feedback: The kidneys are involved in many physiologic processes that influence other body systems beyond the urinary system.
2. What is the main functional unit of the kidneys?
- Nephron
- Glomerulus
- Renal pelvis
- Renal cortex
Take a moment to think about the answer to this question, and then select the button to check your answer.
Correct Answer
Compare your answer to the correct answer below:
A. Nephron
Feedback: The processing of blood and formation of urine occurs in the nephrons. Each kidney has approximately 1 million nephrons.
3. Match each aspect of urine production to the most appropriate description.
- Glomerular filtration
- Tubular reabsorption
- Tubular secretion
Step in which substances are selectively returned to the blood
Step in which substances selectively pass from blood into the filtrate
First step in urine formation
Take a moment to think about the answer to this question, and then select the button to check your answer.
Correct Answer
Compare your answer to the correct answer below:
- Glomerular filtration
- Tubular reabsorption
- Tubular secretion
2 Step in which substances are selectively returned to the blood
3 Step in which substances selectively pass from blood into the filtrate
1 First step in urine formation
Feedback: The end result of these steps is the formation of urine.
Lesson 2. Measuring Renal Function

Patient Perspective: Alan
Dr Thompson explains that he wants to evaluate how well Alan's kidneys are working, so he's going to order some tests.
This lesson examines how renal function is measured.
Lesson Introduction
Measuring renal function provides essential information about how well the kidneys are working. This lesson focuses on how renal function can be assessed and describes the tests that are performed on blood and urine.
Learning Objectives
After completing this lesson, you will be able to:
- Describe the use of serum creatinine (SCr) as a marker of kidney function
- Describe the use of glomerular filtration rate (GFR) as a marker of kidney function
- Identify ways in which the GFR may be estimated
- Explain how urine testing may be used to assess kidney function
Overview of Renal Function Tests and Measurements
Renal function is evaluated by analyzing the composition of the blood and urine to determine how well the kidneys are functioning:
- Serum testing (tests performed on blood): includes assessment of SCr level and blood urea nitrogen (BUN)
- Urine testing includes urinalysis, which measures the amount of protein and other substances in the urine
Clinical Connection: Understanding How Renal Function is Evaluated
There are several methods used to evaluate renal function. These tests and measurements may be used in a number of ways, both in the general population and in patients with HBV infection:
- Screening for renal disease in patients with risk factors
- Diagnosis and evaluation of patients with renal disease
- Monitoring patients who have kidney disease or who are being treated with medications that may require dose adjustments
Serum Creatinine Level
Creatinine is a normal waste product of skeletal muscle metabolism that is formed by the breakdown of creatine phosphate in muscle fibers. The amount of creatinine produced each day is proportional to the body's overall muscle mass. Creatinine is produced at a relatively constant rate each day, and is filtered from the blood by the kidneys and excreted in the urine.
In healthy individuals the amount of creatinine in the blood, referred to as the SCr level, remains consistent from day to day. When renal function is compromised the ability to clear creatinine is impaired and SCr levels will rise. Serum creatinine levels >1.3 mg/dL in men and >1 mg/dL in women are usually abnormal.
Clinical Connection: Significance of Serum Creatinine Levels
Serum creatinine levels are important because they are used to estimate GFR, a key measure of renal function.
Glomerular Filtration Rate
Glomerular filtration rate is the volume of blood filtered through the kidneys per minute. It reflects the average filtration rate of each nephron multiplied by the number of nephrons in both kidneys.
- Glomerular filtration rate is expressed as milliliters (mL) of plasma filtered per minute. Because kidney size is proportional to body size, normal GFR varies depending on the size of the individual. Therefore, GFR may be adjusted for body surface area (BSA) using 1.73 m2 BSA as a constant.
- GFR is affected by age, sex, body weight, and race. It tends to be lower in older patients, females, and those with lower body weight, and tends to be higher in blacks than whites.
- In young adults normal GFR is approximately 120–130 mL/min/1.73 m2 and declines with age. In most healthy people, normal GFR is at least 90 mL/min/1.73 m2.
A decrease in GFR indicates that the kidneys are less able to remove wastes from the body.
GFR is used clinically to measure the degree of renal dysfunction, the progression of established disease, or both. It is usually accepted as the best overall index of kidney function.
Assessment of GFR
The gold standard for the measurement of GFR is measuring the renal clearance of an ideal filtration marker. An ideal filtration marker is one that it is filtered by the kidneys but not secreted, reabsorbed, or metabolized, so that the amount of the marker excreted in the urine precisely reflects the amount filtered by the kidneys; for example, an exogenous marker such as inulin. This technique requires continuous intravenous infusion of the exogenous marker and multiple, timed urine collections, and is cumbersome and not widely available. Alternative exogenous markers are iohexol and iothalamate.
Alternatively, urinary clearance of an endogenous filtration marker, such as creatinine, can be calculated using timed urine collection (eg, 24-hour urine collection) and a single serum measurement. However, this technique is also cumbersome and is not often used in clinical practice.
More commonly, GFR is estimated using an endogenous filtration marker, such as SCr, along with other variables, such as the patient's gender, age, weight, and race. The result is referred to as estimated GFR (eGFR).
Estimating GFR Using Serum Creatinine
Serum creatinine is often used to estimate GFR because it tends to be produced by the muscles and excreted in the urine at a relatively consistent rate, such that blood levels are normally fairly constant. There is an inverse relationship between SCr and GFR; a higher SCr level generally correlates with a reduced GFR.
The relationship between SCr and GFR is not perfectly linear, however, and there are a number of factors that can affect SCr concentrations (see Table 1). Serum creatinine levels can also rise in individuals who experience a decrease in muscle mass, vary their dietary meat intake, or consume creatinine supplements.
Table 1. Factors Affecting Serum Creatinine Concentration
| Factors | Effect on Creatinine | Mechanism |
|---|---|---|
| Age | Decrease | Reduced creatinine generation caused by age-related decline in muscle mass |
| Female | Decrease | Reduced creatinine generation caused by reduced muscle mass |
| African American/black | Increase | Higher creatinine generation caused by higher average muscle mass in African Americans |
| Diet | ||
| Vegetarian | Decrease | Decrease in creatinine generation |
| Ingestion of cooked meats and creatinine supplements | Increase | Transient increase in creatinine generation |
| Body habitus | ||
| Muscular | Increase | Increased muscle generation caused by increased muscle mass and/or increased protein intake |
| Malnutrition, muscle wasting, amputation | Decrease | Reduced creatinine generation caused by reduced muscle mass and/or reduced protein intake |
| Obesity | No change | Excess mass is fat, not muscle mass, and does not contribute to increased creatinine generation |
| Medications | ||
| Trimethoprim, cimetidine, fibric acid derivatives other than gemfibrozil | Increase | Reduced tubular secretion of creatinine |
| Keto acids, some cephalosporins | Increase | Interference with alkaline picrate assay for creatinine |
Formulas for estimating GFR using SCr
A number of mathematical equations have been developed to estimate GFR using SCr; these models rely on the inverse relationship between SCr and GFR, along with adjustment factors for measurable determinants of SCr concentration (eg, age, sex, body size, race).
In adult patients, 3 formulas are commonly used for estimating eGFR based on SCr: the Cockcroft-Gault formula, the Modification of Diet in Renal Disease (MDRD) equation, and the Chronic Kidney Disease Epidemiology (CKD-EPI) formula:
Clinical Connection: Interpreting Estimated Glomerular Filtration Rate Results
When interpreting eGFR results it is important to know which formula was used. The Cockcroft-Gault formula is most commonly used to determine the need for dose adjustments of renally excreted drugs, as studies of medications used in renal failure have traditionally utilized this equation.
- The Cockcroft-Gault formula uses age, SCr, and lean body weight to estimate creatinine clearance (CrCl) rate in milliliters per minute.
- The MDRD equation uses age, SCr, gender, and race (African American/black vs non‑African American/black).
- The MDRD equation was reexpressed in 2005 to be used with SCr measurements from clinical laboratories that have recalibrated their assays traceable to a standardized assay.
- The CKD-EPI formula provides a lower sensitivity but a higher specificity for detecting a GFR less than 60 mL/min/1.73 m2, and it may be more useful in evaluating patients with normal or near-normal kidney function.
These formulas, and their advantages and disadvantages, are listed in Table 2.
Table 2. Creatinine-Based Glomerular Filtration Rate Estimation Equations
| Formula | Equation | Advantages | Disadvantages |
|---|---|---|---|
| Cockcroft-Gault | ([140‑age] x weight)/(72 x SCr) Multiply by 0.85 if female | Popular because of its simple mathematical formulation and bedside applicability | Known to overestimate GFR because of tubular secretion of creatinine, which is not adjustable Not applicable in cases of AKI |
| Abbreviated MDRD equation | 186 x (SCr‑1.154) x (age‑0.203)2 Multiply by 0.742 if female and by 1.210 if black | Includes adjustments for African American/black ethnicity Reexpressed equation is based on standardized creatinine assay More accurate than GFR estimates obtained by Cockcroft-Gault equation | There is no adjustment for other ethnicities A calculator is required Not applicable in cases of AKI |
| Reexpressed MDRD equation | 175 x (SCr‑1.154) x (age‑0.203) | ||
| CKD-EPI | 141 x minimum (SCr/κ, 1)α x maximum (SCr/κ, 1)‑1.200 x 0.993age 3 κ = 0.7 for females and 0.9 for males α = ‑0.329 for females and ‑0.411 for males | Has a higher specificity for detecting a GFR <60 mL/min/1.73 m2 and may be more useful in evaluating patients with normal or near-normal kidney function. | A calculator is required Not applicable in cases of AKI |
AKI, acute kidney injury; CKD-EPI, Chronic Kidney Disease Epidemiology; GFR, glomerular filtration rate; MDRD, Modification of Diet in Renal Disease; SCr, serum creatinine.
Blood Urea Nitrogen
Another test that may be used as a measure of renal function is BUN.
BUN is a by-product of the breakdown of amino acids and of ingested protein and the body's own (endogenous) protein. The level of urea in the blood can provide a rough estimate of kidney function, and elevated BUN levels (>20 mg/dL) can indicate impaired renal function.
Urine Testing
Urinalysis is also used to evaluate renal function. Normal urine consists of water (~95% of total urine volume), electrolytes, solutes derived from cellular metabolism, and exogenous substances, such as drugs.
If disease alters body metabolism or kidney function, the normal constituents of urine may appear in abnormal amounts or substances not normally found in urine may be present.
Proteinuria and albuminuria
Normal urine is virtually protein-free, because the filtration process keeps large molecules, such as protein, in the blood. If kidney function is impaired, however, protein may appear in the urine (referred to as proteinuria).
Specifically, the presence of the protein albumin in the urine (albuminuria) is used to diagnose and monitor kidney disease.
Depending on the degree and duration, proteinuria or albuminuria may indicate kidney injury or impairment.
Urine albumin-to-creatinine ratio
The simplest way to measure urine albumin is by using a dipstick test. However, the presence and level of albuminuria vary according to urine concentration, so a single dipstick test may not be reliable if urine is more or less concentrated than average.
The urine albumin-to-creatinine ratio (UACR) may be calculated to diagnose and monitor kidney disease. The UACR is the urine albumin concentration divided by the urine creatinine concentration. Compared to a simple dipstick test, this measurement can more accurately detect albuminuria in a single urine sample. A UACR >30 mg/g is considered a marker for CKD.

Patient Perspective: Alan
Dr Thompson performs some standard blood tests, including measuring Alan's serum creatinine levels and using them to estimate his glomerular filtration rate. He also looks for protein in Alan's urine.
Lesson Summary
- Renal function is mainly evaluated using serum testing or urinalysis.
- Creatinine levels in the blood can be used as an indicator of renal function. Elevated SCr levels (>1.3 mg/dL in men and >1 mg/dL in women) may indicate renal dysfunction.
- Glomerular filtration rate is the volume of blood filtered through the kidneys each minute, and is accepted as the best overall index of kidney function.
- Normal GFR varies depending on age, sex, body weight, and race, and tends to be lower in older patients, females, and those with lower body weight.
- In clinical practice GFR is usually estimated using an equation that considers the patient's SCr level, such as the Cockcroft-Gault formula, the MDRD equation, or the CKD-EPI formula.
- The Cockcroft-Gault formula is the most common formula used for dose adjustments of renally excreted drugs.
- Levels of BUN, a by-product of the breakdown of amino acids and protein, may also be used to assess renal function.
- In addition to these serum tests, urine may be analyzed to detect the presence of protein, typically albumin, which is not usually found in the urine in healthy individuals.
Self-Check Questions
1. Which of the following statements about creatinine are correct? Select all that apply.
- Elevated levels of creatinine may indicate renal dysfunction.
- Creatinine is typically measured in the blood rather than the urine.
- A patient's creatinine levels can be used to estimate the GFR.
- Creatinine levels tend to be higher in the elderly, in females, and in individuals with low body weight.
Take a moment to think about the answer to this question, and then select the button to check your answer.
Correct Answer
Compare your answer to the correct answer below:
A. Elevated levels of creatinine may indicate renal dysfunction.
B. Creatinine is typically measured in the blood rather than the urine.
C. A patient's creatinine levels can be used to estimate the GFR.
Feedback: Creatinine is a by-product of the breakdown of muscle that occurs during normal metabolism. The amount of ceatinine produced each day is normally excreted via the kidney, so serum creatinine levels tend to remain constant. Elevated creatinine levels may signal impaired renal function.
2. Complete the following sentence.
A patient's creatinine clearance rate is roughly equivalent to his or her _______________________.
Take a moment to think about the answer to this question, and then select the button to check your answer.
Correct Answer
Compare your answer to the correct answer below:
A patient's creatinine clearance rate is roughly equivalent to his or her glomerular filtration rate.
Feedback: Creatinine clearance—the rate at which creatinine is cleared from the serum by the kidneys—correlates with GFR. Glomerular filtration rate and creatinine clearance are both indicators of a patient's renal function.
3. Complete the following sentence.
A high level of the protein _______________________ in the blood is an indicator of impaired renal function.
Take a moment to think about the answer to this question, and then select the button to check your answer.
Correct Answer
Compare your answer to the correct answer below:
High levels of the protein albumin in the blood are an indicator of impaired renal function.
Feedback: In healthy kidneys, proteins normally remain in the blood and are not excreted in the urine. Albuminuria may indicate impaired kidney function.
Lesson 3. Kidney Disease

Patient Perspective: Alan
Dr Thompson tells Alan that his test results show evidence of renal impairment, and that he likely has chronic kidney disease. Alan shakes his head and says that can't be true. As far as he knows he doesn't have any symptoms, and no one has ever told him he has a problem with his kidneys.
Dr Thompson explains that it's quite possible for patients with kidney disease to be asymptomatic, especially in its early stages.
This lesson discusses the major types and classifications of kidney disease.
Lesson Introduction
Kidney disease is a general term for any abnormality of kidney structure or function that impacts health.
This lesson discusses how kidney disease is classified, the distinction between acute kidney injury (AKI) and CKD, and how CKD is staged.
Learning Objectives
After completing this lesson, you will be able to:
- Define kidney disease
- Distinguish between acute kidney injury (AKI) and chronic kidney disease (CKD)
- Describe how CKD is staged
Overview of Kidney Disease
Kidney disease refers to any abnormality of kidney structure or function with implications for the health of an individual. Kidney disease can occur abruptly and can either resolve or become chronic.
Acute Kidney Injury
Acute kidney injury is a syndrome characterized by an abrupt decrease in kidney function over a period of hours to days, resulting in the buildup of creatinine and other waste products in the blood.
Generally, acute kidney injury can occur when:
- There is a condition that interferes with blood flow to the kidneys.
- There is direct damage to the kidneys (eg, trauma, a toxic substance).
- The ureters (the tubes that drain urine from the kidneys) become blocked and interfere with removal of wastes from the kidneys.
Acute kidney injury can range from an impairment in renal function to acute renal failure. It is a broad clinical syndrome that encompasses various etiologies, including specific kidney diseases (eg, acute interstitial nephritis), nonspecific conditions such as ischemia or toxic injury, and conditions outside the kidney that adversely affect renal function.
Depending on the cause, AKI may be reversible.
Definition
According to the organization Kidney Disease: Improving Global Outcomes (KDIGO), AKI may be defined as any of the following:
- Increase in SCr by ≥0.3 mg/dL within 48 hours
- Increase in SCr to ≥1.5 times baseline levels that is known or presumed to have occurred within the past 7 days
- Urine volume <0.5 mg/kg for 6 hours
Etiology
As previously noted, AKI has a range of causes and contributors, including diseases of the kidney, extrarenal conditions, and nonspecific causes. Nonspecific causes include exposure to nephrotoxic medications that are cleared by the kidneys, radiocontrast agents, critical illness, and major surgery.
Causes of AKI can be classified as prerenal, intrinsic, or postrenal. (See Table 3.)
Table 3. Causes of Acute Kidney Injury
| Type of Cause | Description |
|---|---|
| Prerenal | Associated with decreased renal perfusion due to:
|
| Intrinsic | Associated with a process within the kidneys
|
| Postrenal | Associated with a process downstream from the kidneys that interferes with drainage of urine from the kidneys
|
Factors That Increase Susceptibility to Acute Kidney Injury
The kidneys can withstand several insults without significant damage. Some patients, however, are more susceptible to AKI, specifically:
- Females
- African Americans/blacks
- The elderly
- Those with CKD or chronic diseases of the heart, lung, or liver
- Those with diabetes mellitus, cancer, or anemia
- Dehydration or volume depletion
Chronic Kidney Disease
Chronic kidney disease refers to any illness in which kidney function remains diminished for at least 3 months without returning to normal. CKD is a general term for heterogeneous disorders affecting kidney structure or function, and its clinical presentation may vary depending on cause, severity, and the rate of progression. As such, CKD encompasses a spectrum of severity that can range from kidney damage with preserved renal function to impaired kidney function to kidney failure.
According to estimates from the Centers for Disease Control and Prevention, over 10% of adults in the United States—more than 20 million people—may have CKD of varying levels of severity. The risk for developing CKD increases after age 50 and is most common among adults older than 70.
The clinical course of CKD varies. It often evolves over decades, and patients are often asymptomatic during the earlier stages of the disease. However, some patients progress to kidney failure within months. In its earlier stages, CKD may be reversible.
Definition
Chronic kidney disease is generally defined as either the presence of kidney damage or a GFR less than 60 mL/min/1.73 m2 for at least 3 months.
Etiology of Chronic Kidney Disease
The causes of CKD may include:
- Systemic disease or other factors that cause kidney damage. This includes conditions that damage the renal vasculature, such as diabetes or hypertension, and conditions that damage the renal tissue, such as HBV infection.
- Intrinsic kidney diseases that originate in or are confined to the kidneys, such as glomerulonephritis.
Risk factors for CKD are discussed in Lesson 4.
Staging of Chronic Kidney Disease
CKD may be classified into 5 stages based on the patient's eGFR or need for dialysis. (See Table 4.)
Table 4. Staging of Chronic Kidney Disease Based on Glomerular Filtration Rate
| Stage | Description | GFR (mL/min/1.73 m2) | Actiona |
|---|---|---|---|
|
| At increased risk | ≥90 (with CKD risk factors) | Screening, CKD risk reduction |
| 1 | Kidney damage with normal or increased GFR | ≥90 | Diagnosis and treatment Treatment of comorbid conditions Slowing progression CVD risk reduction |
| 2 | Kidney damage with mild or decreased GFR | 60–89 | Estimating progression |
| 3 | Moderate decreased GFR | 30–59 | Evaluating and treating complications |
| 4 | Severe decreased GFR | 15–29 | Preparation for kidney replacement therapy |
| 5 | Kidney failure | <15 (or dialysis) | Replacement (if uremia present) |
CKD, chronic kidney disease; CVD, cardiovascular disease GFR; glomerular filtration rate.
Chronic kidney disease is defined as either kidney damage or GFR <60 mL/min/1.73 m2 for ≥3 months. Kidney damage is defined as pathologic abnormalities or markers of damage, including abnormalities in blood or urine tests or imaging studies.
aIncludes actions from preceding stages.
At one end of the spectrum, patients at stage 1 have kidney damage in the presence of normal or even above-normal GFR (≥90 mL/min/1.73 m2). These patients may have few or no symptoms. At the other end, patients at stage 5 have GFR <15 mL/min/1.73 m2 or are receiving dialysis; these patients are considered to have end-stage renal disease (ESRD).
Clinical Connection: End-Stage Renal Disease
ESRD refers to the advanced kidney disease that has advanced to the point at which the kidneys are no longer able to remove wastes and function adequately to sustain life. Patients with ESRD typically require hemodialysis or kidney transplantation (collectively referred to as renal replacement therapy) to sustain life.
Progression of Chronic Kidney Disease
The rate at which kidney function declines and patients progress through these stages varies. This progression is affected by such factors as the cause of CKD, the presence of albuminuria or proteinuria, the patient's comorbidities, and age.
The presence and level of albuminuria can be combined with the eGFR to better estimate the risk of CKD progression; lower GFR and greater albuminuria are synergistic and are both associated with an increased rate of progression.
Understanding the Relationship Between Acute Kidney Injury and Chronic Kidney Disease
It is important to note that patients may have both AKI and CKD. If AKI does not resolve and renal impairment persists for more than 3 months, the patient may be considered to have CKD. Conversely, AKI may occur in patients who have already been diagnosed with CKD if they experience an abrupt decrease in renal function superimposed on chronic disease. In such patients, AKI may be considered a complication of CKD and may contribute to its progression.
Clinical Presentation
In its earliest stages, CKD may be asymptomatic. As CKD progresses, patients may present with various signs and symptoms caused by progressive impairment in kidney function or due to complications of CKD.
Signs and symptoms associated with mild to moderate renal insufficiency include:
- Nocturia
- Lassitude
- Fatigue
- Anorexia
- Decreased mental acuity
Patients with more severe renal insufficiency may experience the following types of symptoms:
- Neuromuscular symptoms, such as muscle twitches, peripheral sensory and motor neuropathies, muscle cramps, and seizures
- Gastrointestinal symptoms, such as anorexia, nausea, vomiting, weight loss, stomatitis, and unpleasant taste in the mouth
- Constitutional symptoms, such as yellow-brown skin, pruritus, and generalized tissue wasting (a prominent feature of chronic uremia)
Potential Complications
Over time, patients with CKD may present with complications arising from impaired kidney function and its physiologic effects throughout the body. These may include:
- Electrolyte and acid-base disturbances
- Hypertension
- Malnutrition or poor nutritional health
- Anemia due to lack of erythropoietin
- Uremia
- Hypoalbuminemia
- Vascular calcification
- Cardiovascular disease
- Secondary hyperparathyroidism
- Lipid abnormalities
It is important to note that CKD may be associated with mineral abnormalities, including hyperphosphatemia, hypocalcemia, and vitamin D deficiency.
Clinical Connection: Phosphate and CKD
The kidneys help regulate levels of phosphate in the body by excreting excess phosphate. Elevated levels of phosphate in the blood (hyperphosphatemia) are often therefore an indicator of impaired kidney function and may occur in people with CKD. Serum phosphate levels may be monitored in patients with kidney disease.

Patient Perspective: Alan
Dr Thompson shares Alan's test results with him. Alan's serum creatinine level is 1.25 mg/dL, and his estimated GFR is 79 mL/min. His dipstick test is positive for proteinuria, and his serum phosphate levels are 3 mg/mL. Based on Alan's test results, Dr Thompson says that he is in the early stages of chronic kidney disease.
Dr Thompson explains that chronic kidney disease can develop over time, and that most patients are asymptomatic until complications develop. He notes the importance of monitoring his kidney disease.
Lesson Summary
- Kidney disease is a nonspecific term for any derangement in kidney structure or function that can occur abruptly and either resolve or become chronic.
- Acute kidney injury—an abrupt decrease in kidney function over a period of hours to days—encompasses a variety of etiologies and a range of severities.
- It can occur when there is a condition that interferes with blood flow to the kidneys, when there is direct damage to the kidneys, or when the ureters are blocked, interfering with waste removal from the kidneys.
- Depending on the cause, AKI may be reversible.
- Chronic kidney disease is any illness in which kidney function remains diminished for at least 3 months without returning to normal.
- It is generally defined as the presence of kidney damage or GFR <60 mL/min/1.73 m2 for at least 3 months.
- It can range from kidney damage with preserved function to impaired function to kidney failure.
- According to some estimates, more than 10% of adults in the United States have CKD of varying levels of severity.
- CKD is classified into 5 stages based on the patient's GFR and whether the patient requires dialysis. Patients at the lowest stage (1) have kidney damage with normal or increased GFR. Patients at the highest stage (5) are in kidney failure and have a GFR <15 mL/min/1.73 m2 or require dialysis.
- The rate of progression through these stages varies.
- Patients in the early stages of CKD may be asymptomatic.
- Patients with mild to moderate renal insufficiency may have a variety of symptoms, such as nocturia, lassitude, anorexia, and decreased mental acuity.
- More severe renal insufficiency may be associated with neuromuscular symptoms (eg, muscle cramps, seizures), gastrointestinal symptoms (eg, nausea, vomiting), or constitutional symptoms (eg, pruritus, yellow-brown skin tone).
- Chronic kidney disease may give rise to complications affecting multiple body systems, including hypertension, anemia, mineral and bone disorders, and cardiovascular disease.
- Serum phosphate levels may be elevated in patients with impaired kidney function, and may be monitored in patients with kidney disease.
Self-Check Questions
1. Name 3 general causes of acute kidney injury.
Take a moment to think about the answer to this question, and then select the button to check your answer.
Correct Answer
Compare your answer to the correct answer below:
- Conditions that interfere with blood flow to the kidneys
- Direct damage to the kidneys
- Blockage of the ureters that interferes with removal of wastes from the kidneys
Feedback: The causes of acute kidney injury can be classified as prerenal, intrinsic renal, and postrenal causes.
2. Which of the following statements about AKI are correct? Select all that apply.
- It is always irreversible.
- It can be caused by agents that are toxic to the kidneys.
- It is defined as impaired kidney function that lasts at least 3 months.
- Those who have CKD are more susceptible to AKI.
Take a moment to think about the answer to this question, and then select the button to check your answer.
Correct Answer
Compare your answer to the correct answer below:
B. It can be caused by agents that are toxic to the kidneys.
D. Those who have CKD are more susceptible to AKI.
Feedback: AKI can be caused by a variety of factors within and outside the kidneys, including nephrotoxic substances. Depending on the cause and degree of damage, AKI is sometimes reversible.
3. How is chronic kidney disease defined?
Take a moment to think about the answer to this question, and then select the button to check your answer.
Correct Answer
Compare your answer to the correct answer below:
CKD is generally defined as either the presence of kidney damage or a GFR <60 mL/min/1.73 m2 for at least 3 months.
Feedback: CKD can develop gradually or it can result from AKI that does not resolve.
4. Which of the following words or phrases best completes the following sentence?
Chronic kidney disease is classified by stage based on the patient's _______________________.
Take a moment to think about the answer to this question, and then select the button to check your answer.
Correct Answer
Compare your answer to the correct answer below:
B. GFR
Feedback: The severity of CKD is staged based on the patient's GFR and whether there is a need for dialysis. Patients at stage 1 may have kidney damage with normal or even high GFR, while those at stage 5 have more extensive damage and GFR <15 mL/min/1.73 m2 or may require dialysis.
Lesson 4. Risk Factors for CKD

Patient Perspective: Alan
Alan is surprised. Isn't he too young for kidney problems? He doesn't even know anyone with kidney disease, except an aunt who's on dialysis and maybe one of his other relatives.
This lesson examines CKD risk factors.
Lesson Introduction
There are a number of factors that can increase an individual's risk of developing CKD. Understanding these factors may help clarify why CKD is a growing problem in the United States and other countries.
Learning Objectives
After completing this lesson, you will be able to:
- Identify the risk factors for chronic kidney disease
- Explain why CKD is a growing problem in the United States and elsewhere
Risk Factors for CKD
As you learned in Lesson 3, CKD can result from a variety of illnesses or injury to the kidney that interfere with renal function.
- Diabetes mellitus
- Hypertension
- Cardiovascular disease
- Obesity
- Family history of CKD
- Being over age 60
- African-American/black, Asian, Native American, or Hispanic ethnicity
- Other conditions affecting the kidneys, such as polycystic kidney disease, glomerulonephritis, autoimmune diseases (eg, systemic lupus erythematosus, IgA nephropathy), and kidney cancer
In the United States, the most common cause of CKD is kidney damage caused by diabetes mellitus and hypertension.
Clinical Connection: CKD is a Growing Problem
According to one projection, the number of Americans with CKD aged 30 or older will reach 28 million in 2020 and nearly 38 million in 2030. Some reasons for this increased prevalence include the aging of the American population and with it, decreased kidney function and increased risk for diabetes mellitus and other conditions that fuel the development of CKD.

Patient Perspective: Alan
Dr Thompson notes that Alan has several risk factors for kidney disease, and reviews them with him. Alan is surprised to learn how common chronic kidney disease is.
As Dr Thompson reviews Alan's risk factors, he reminds him of the importance of controlling his hypertension and dyslipidemia, and making sure his blood glucose stays within the normal range.
Lesson Summary
- A variety of risk factors for developing CKD have been identified. In the United States the most common cause of CKD is kidney damage caused by diabetes mellitus and hypertension.
- Other systemic conditions associated with increased risk for developing CKD are cardiovascular disease and obesity.
- Certain demographic factors (eg, increasing age; family history of CKD; African-American/black, Asian, Native American, or Hispanic ethnicity) are also associated with increased risk.
- Conditions that affect the kidneys (eg, polycystic kidney disease, glomerulonephritis, autoimmune diseases, kidney cancer) increase the risk for CKD.
- The number of Americans with CKD aged 30 or older will reach 28 million in 2020 and nearly 38 million in 2030.
- This anticipated increase in prevalence is due to the aging of the American population, accompanied by age-related decline in kidney function and increased prevalence of diabetes mellitus and other conditions that increase the risk for developing CKD.
Self-Check Questions
1. Which of the following are risks factors for chronic kidney disease? Select all that apply.
- Hypertension
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Obesity
- Diabetes mellitus
- Systemic lupus erythematosus
- White ethnicity
- African-American/black ethnicity
- Asian ethnicity
Take a moment to think about the answer to this question, and then select the button to check your answer.
Correct Answer
Compare your answer to the correct answer below:
A. Hypertension
B. Cardiovascular disease
D. Obesity
E. Diabetes mellitus
F. Systemic lupus erythematosus
H. African-American/black ethnicity
I. Asian ethnicity
Feedback: In addition to the risk factors for CKD noted above, others include increasing age, family history of CKD, Native American or Hispanic ethnicity, and other diseases that affect the kidneys, such as polycystic kidney disease.
2. Why is the prevalence of CKD anticipated to increase in the United States?
Take a moment to think about the answer to this question, and then select the button to check your answer.
Correct Answer
Compare your answer to the correct answer below:
The prevalence of CKD is expected to increase due to aging of the American population, which is typically associated with decreased renal function, and because of increased prevalence of diabetes mellitus, hypertension, and other conditions that are risk factors for CKD.
Feedback: In the United States, diabetes mellitus and hypertension are the greatest contributors to CKD.
Lesson 5. Kidney Disease and HBV

Patient Perspective: Alan
There are a number of factors related to Alan's renal status and his chronic hepatitis B infection that Dr Thompson must consider in his treatment plan.
This lesson focuses on how CHB infection can affect the kidneys.
Lesson Introduction
Hepatitis B virus infection can directly affect the kidneys. Knowing how this happens is key to an overall understanding of CHB infection and the considerations that may arise regarding renal health.
Learning Objectives
- After completing this lesson, you will be able to describe how hepatitis B infection can affect the kidneys.
Hepatitis B Virus and the Kidneys
Why is kidney disease a concern in the management of chronic hepatitis B (CHB)? There are 3 potential areas of concerns:
- The hepatitis B virus may itself be linked to specific renal abnormalities, such as glomerulonephritis.
- Some of the medications used in the management of CHB, particularly nucleos(t)ide reverse transcriptase inhibitors (NRTIs), may be associated with nephrotoxicity.
- Patients with renal impairment, such as those with CKD, may have difficulty eliminating certain medications and therefore may require dose adjustments of these medications.
Clinical Connection: Treatment Considerations for Healthcare Providers
When planning and administering treatment for patients with CHB, healthcare providers (HCPs) must be mindful of how HBV infection may affect the patient's kidneys, the potential nephrotoxic effects of some medications, and any dose adjustments that may be needed in the event of impaired renal function.
This lesson will discuss the first point: renal abnormalities linked to HBV infection.
HBV-Associated Renal Disease
HBV infection may injure the kidneys and ultimately lead to kidney disease. The mechanisms by which HBV injures the kidney are complex; generally, however, they may be related to immune reactions causing the deposition of immune complexes in renal tissue, or the effects of cytokines and mediators produced by the immune system on renal tissue.
The renal abnormalities associated with HBV may include:
- Various forms of nephropathy
- Glomerulonephritis
- Conditions associated with glomerular sclerosis

Patient Perspective: Alan
Alan is surprised to learn that CHB infection, in addition to affecting his liver, can also have harmful effects on his kidneys over time.
Lesson Summary
- CHB and renal health are related in 3 ways:
- The hepatitis B disease process itself may injure the kidneys.
- Some medications used to treat CHB (eg, NRTIs) may be associated with nephrotoxicity.
- Those with renal impairment may have difficulty eliminating certain medications and may require dose adjustments.
- The mechanisms by which HBV injures the kidney are complex, and may be related to immune-mediated mechanisms, such as the deposition of immune complexes in renal tissue, or the effects of cytokines and mediators produced by the immune system on renal tissue.
- Renal abnormalities potentially associated with HBV may include:
- Various forms of nephropathy
- Glomerulonephritis
- Conditions associated with glomerular sclerosis
Self-Check Questions
1. What are the 3 ways that CHB and renal health are related?
Take a moment to think about the answer to this question, and then select the button to check your answer.
Correct Answer
Compare your answer to the correct answer below:
- The hepatitis B virus itself may injure the kidneys
- Some medications used to treat CHB may be associated with nephrotoxicity
- People with renal impairment may have difficulty eliminating certain medications and may require dose adjustments
Feedback: These factors should all be considered when planning and administering treatment for patients with CHB.
2. Complete the following sentence.
The mechanisms by which HBV can injure the kidneys may be mediated by the _____________.
Take a moment to think about the answer to this question, and then select the button to check your answer.
Correct Answer
Compare your answer to the correct answer below:
The mechanisms by which HBV can injure the kidneys may be mediated by the immune system.
Feedback: Potential immune-mediated effects leading to kidney injury may be related to deposition of immune complexes in renal tissue and the effect of cytokines and mediators on the renal tissue.
Lesson 6. How Oral Antiviral Treatment in Patients With Chronic Hepatitis B May Affect Kidney Function

Patient Perspective: Alan
As Dr Thompson reviews Alan's treatment plan, he takes into consideration the potential renal effects of certain antiviral medications, as well as how Alan's kidney function might affect his ability to eliminate some medications.
This lesson examines how antiviral medications may affect the kidneys and how the kidneys may influence the use of certain antiviral medications.
Lesson Introduction
When managing patients with CHB, HCPs must consider the potential for nephrotoxic effects associated with some medications and must also be aware of treatment considerations for patients with renal impairment. This lesson discusses mechanisms and risk factors for nephrotoxicity and dosing considerations for certain antiviral medications.
Learning Objectives
Risk Factors for Nephrotoxicity
Several risk factors have been identified that may contribute to the enhanced likelihood of developing kidney injury. These risk factors include:
- Patient-specific factors, such as female sex, older age, the presence of cirrhosis or obstructive jaundice, and underlying acute kidney disease or CKD. Decreased GFR and certain metabolic abnormalities also increase the kidney's vulnerability to nephrotoxicity.
- Kidney-specific factors, such as the high rate of blood delivery to the kidneys, the high metabolic rate of tubular cells, and the tendency for uptake of toxins in the proximal tubules.
- Drug-specific factors, such as prolonged dosing periods and toxin exposure, the inherent direct nephrotoxic effects of some compounds, and the potential for enhanced nephrotoxicity with certain combinations.
After completing this lesson, you will be able to:
- Describe how certain medications used in the management of chronic hepatitis B may affect the kidneys
- Explain why dosages of certain medications may need to be adjusted for patients with renal impairment
Antiviral Medications and the Kidney
When managing patients with CHB, HCPs must consider the potential for nephrotoxic effects associated with certain medications that may cause nephrotoxicity.
Another important consideration for HCPs is that patients with renal impairment, such as those with CKD, may have difficulty eliminating certain medications and therefore may require dose adjustments.
This lesson discusses these considerations.
Understanding Nephrotoxicity
One of the major functions of the kidneys is the metabolism and excretion of exogenously administered therapeutic and diagnostic agents. As a result of this role, the kidney is susceptible to various forms of injury from nephrotoxic drugs.
Humans may be exposed to a variety of potential nephrotoxic substances. Examples of agents with known nephrotoxic potential include certain antimicrobial agents, chemotherapeutic agents, analgesics, immunosuppressive agents, and certain antiviral agents.
Significant renal exposure to potential nephrotoxins occurs due to the high rate of drug and toxin delivery to the kidney. This is a result of the high rate of blood flow to the kidney, which approaches 25% of the cardiac output. Cells in the proximal tubules of the kidneys are particularly vulnerable to damage due to the enhanced uptake of potential toxins and drugs that occurs there.
Implications for Management of CHB
Specific attention should therefore be paid to renal tubule function in patients with CHB, including baseline assessment of phosphorus and other indicators of tubular function, and to follow-up testing in patients receiving antivirals with the potential for nephrotoxicity. Attention should also be given to factors that may increase a patient's risk for nephrotoxicity.
Renal Effects of Nucleos(t)ide Reverse Transcriptase Inhibitors
Some NRTIs (eg, adefovir, TDF) have been associated with renal toxicity, related to an accumulation of nucleotide metabolites in renal tubular cells. This may manifest as dysfunction of the proximal renal tubule (referred to as proximal renal tubulopathy [PRT] due to the accumulation of drug at that site). PRT may interfere with the ability of the proximal tubule to reabsorb phosphate, potentially leading to hypophosphatemia and osteomalacia.
In rare cases, PRT can progress to Fanconi syndrome. This syndrome is characterized by abnormal functioning of the proximal tubules of the kidney, resulting in the loss of amino acids, glucose, phosphates, and urates in abnormal concentrations in the urine. Polyuria, osteomalacia, and growth failure are common consequences of Fanconi syndrome.
Antiviral Drug Therapy in Patients With CKD
Now let's look at the considerations associated with the use of antiviral therapy in patients with CKD.
CKD can interfere with the ability of the kidney to filter and excrete some medications. Accordingly, the pharmacokinetics of drugs that are renally excreted may be significantly modified in patients with CKD due to alterations in metabolism and the reduced ability to eliminate medications from the body.
Dose adjustments (typically dose reduction) for certain antiviral medications may be necessary depending on the patient's level of renal function, usually expressed as the CrCl rate or estimated GFR (eGFR). At the same time, care must be taken to avoid adjusting the dose to a subtherapeutic level. Clinicians should follow dosing recommendations in the product labeling of each respective product.
Clinical Connection: Measuring Baseline Renal Function
Because kidney disease may go unrecognized in its earlier stages, it is important to measure baseline renal function in patients with CHB before beginning antiviral therapy and to monitor renal function thereafter.
Dose Adjustments in Patients With CKD
Long-term nucelos(t)ide analogue therapy is generally recommended for all treatment-eligible patients with CHB regardless of the presence of renal disease. However, as previously noted, dose adjustments (typically dose reductions) of certain agents may be necessary for some patients with CKD, depending on the degree of renal impairment.
The KDIGO has published recommendations for dose adjustments of nucleos(t)ide analogues and interferon in patients with CKD. (See Table 5.) Note that these recommendations are for a global audience and reflect dosages of agents used outside the United States.
Recommendations for Patients With HBV–Associated Renal Disease
What about patients who have renal disease as a consequence of their CHB? KDIGO guidelines for CKD state that these patients should be treated according to standard clinical practice guidelines for HBV infection, with dosing of interferon or nucleos(t)ide analogues adjusted to the degree of kidney function. (See Table 5.)
Table 5. Recommendations for Dose Adjustments of Nucleos(t)ide Analogues and Interferon in Patients With CKD
| CrCl (ml/min) | Lamivudine | Telbivudine | Adefovir | Entecavira | TDFb | Pegylated interferon α-2α |
|---|---|---|---|---|---|---|
| >50 | 100 mg/day | 600 mg/day | 10 mg/day | 0.5 mg/day | 300 mg/day | 180 μg SC/week |
| 30–49 | 100 mg first dose, then 50 mg/day | 600 mg/day 2 | 10 mg/day 2 | 0.25 mg/day | 300 mg/day 2 | 135 μg SC/week |
| 15–29 | 35 mg first dose, then 25 mg/day | 600 mg/day 3 | 10 mg/day 3 | 0.15 mg/day | 300 mg/day 2-3 |
|
| 5–14 | 35 mg first dose, then 15 mg/day | 600 mg/day 3 | 10 mg/day 3c | 0.05 mg/dayc | 300 mg/weekc |
|
| <5 | 35 mg first dose, then 10 mg/day | 600 mg/day 4 | 10 mg/week following hemodialysisd | 0.5 mg/week following hemodialysisd | 300 mg/week following hemodialysisd |
|
CrCl, creatinine clearance; SC, subcutaneous; TDF, tenofovir disoproxil fumarate.
aRecommendations only for nucleos(t)ide analogue–naive patients.
bIn the United States, the recommended dose of TDF for patients aged ≥12 years is 300 mg/day.
cRecommendation only for CrCl x 10 ml/min.
dOnly for patients on hemodialysis.

Patient Perspective: Alan
As Dr Thompson considers the pharmacologic options for treating Alan's CHB, he considers their potential effects on the kidneys and whether dose adjustments are necessary for renally impaired patients. He explains to Alan that some antiviral medications can have nephrotoxic effects, and that certain agents may require dose adjustments for some patients.
Lesson Summary
- When managing patients with CHB, clinicians must consider the potential for nephrotoxic effects associated with certain medications, as well as whether the patient requires a dose adjustment due to renal impairment.
- The kidney is susceptible to various forms of injury from nephrotoxic drugs and other substances. The cells in the tubular structures of the kidney may be especially vulnerable to nephrotoxins.
- Some NRTIs have been associated with renal toxicity due to accumulation of nucleotides in renal tubular cells.
- This may manifest as proximal renal tubulopathy (PRT), which may interfere with the ability of the proximal tubule to reabsorb phosphate.
- Consequences may include hypophosphatemia and osteomalacia.
- In rare cases, PRT may progress to Fanconi syndrome.
- Clinicians should pay special attention to renal tubule function in patients with CHB, including baseline assessment of renal function as well as phosphorus levels and other indicators of tubular function.
- When receiving certain medications, patients with CHB and coexisting CKD may require dose adjustments. These dose adjustments are typically based on a patient's CrCl rate or estimated GFR.
- Antiviral therapy should not be withheld for patients who already have experienced renal complications due to HBV.
- These patients should be treated according to standard clinical practice guidelines for HBV, with dose adjustments where recommended.
Self-Check Questions
1. Which type of renal abnormality has been associated with the use of certain NRTIs?
- Sclerosis of the peritubular capillaries
- Deposition of immune complexes in the glomeruli
- Accumulation of nucleotide metabolites in renal tubular cells
- Increased formation of crystals and stones that block urine flow
Take a moment to think about the answer to this question, and then select the button to check your answer.
Correct Answer
Compare your answer to the correct answer below:
C. Accumulation of nucleotide metabolites in renal tubular cells
Feedback: This may lead to dysfunction of the proximal renal tubule (proximal renal tubulopathy) and, in rare cases, Fanconi syndrome.
2. Complete the following sentence.
The pharmacokinetics of certain ______________excreted drugs may be altered in patients with CKD and a dose adjustment, usually a ___________, may be recommended.
Take a moment to think about the answer to this question, and then select the button to check your answer.
Correct Answer
Compare your answer to the correct answer below:
The pharmacokinetics of certain renally excreted drugs may be altered in patients with CKD and a dose adjustment, usually a reduction, may be recommended.
Feedback: Guidelines recommend that treatment-eligible patients with CHB should receive long-term nucelos(t)ide analogue therapy, regardless of the presence of renal disease. Depending on the individual agent and the patient's renal status, dose adjustments may be recommended.
References Cited
American Kidney Fund web site. Are you at risk? http://www.kidneyfund.org/prevention/are-you-at-risk. Accessed June 2, 2016.
Baumgarten M, Gehr T. Chronic kidney disease: detection and evaluation. Am Fam Physician. 2011;84(10):1138-1148.
Buti M, Gane E, Seto W-K, et al. A phase 3 study of tenofovir alafenamide compared with tenofovir disoproxil fumarate in patients with HBeAg-negative, chronic hepatitis B: week 48 efficacy and safety results. Abstract GS06. J Hepatol. 2016;64;S133–S158.
Buti M, Gane E, Seto WK, et al. A phase 3 study of tenofovir alafenamide compared with tenofovir disoproxil fumarate in patients with HBeAg-negative, chronic hepatitis B: week 48 efficacy and safety results. EASL Barcelona. 2016. Slide deck (provided by Gilead).
Centers for Disease Control and Prevention. National chronic kidney disease fact sheet, 2014. http://www.cdc.gov/diabetes/pubs/pdf/kidney_factsheet.pdf. Accessed June 1, 2016.
Chan HLY, Fung S, Seto WK, et al. A phase 3 study of tenofovir alafenamide compared with tenofovir disoproxil fumarate in patients with HBeAg-positive, chronic hepatitis B: week 48 efficacy and safety results. EASL Barcelona. 2016. Slide deck (provided by Gilead).
Chan HLY, Fung S, Seto WK, et al. A phase 3 study of tenofovir alafenamide compared with tenofovir disoproxil fumarate in patients with HBeAg-positive, chronic hepatitis B: week 48 efficacy and safety results. Abstract GS12. J Hepatol. 2016;64;S133-S158.
Deray G, Buti M, Gane E, et al. Hepatitis B virus infection and the kidney: renal abnormalities in HBV patients, antiviral drugs handling, and specific follow-up. Adv Hepatol. 2015. https://www.hindawi.com/archive/2015/596829/.
Fung J, Seto W-K, Lei C-L, Yuen M-F. Extrahepatic effects of nucleoside and nucleotide analogues in chronic hepatitis B treatment. J Gastroenterol Hepatol. 2014;29(3):428-434.
Gilead Sciences. Data on file.
Gupta SK, Winston JA, Eustace JA, et al. Guidelines for the management of chronic kidney disease in HIV-Infected patients: recommendations of the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2005;40(11):1559-1585.
Hoerger TJ, Simpson SA, Yarnoff BO, et al. The future burden of CKD in the United States: a simulation model for the CDC CKD Initiative. Am J Kidney Dis. 2015;65(3):403-411.
Inker LA, Fan L, Levey AS. Assessment of renal function. In: Johnson RJ, Fehally J, Floege J, eds. Comprehensive Clinical Nephrology. 5th ed. Philadelphia, PA: Saunders Elsevier; 2015.
Kidney Disease Improving Global Outcomes® (KDIGO). KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2013;3(suppl 1):1-150.
Kidney Disease Improving Global Outcomes® (KDIGO). KDIGO clinical practice guideline for acute kidney injury. Kidney Int. 2012;2(suppl 1):1-138.
Lascano ME, Poggio ED. At: Cleveland Clinic Center for Continuing Education. Kidney function assessment by creatinine-based estimation equations. http://www.clevelandclinicmeded.com/medicalpubs/diseasemanagement/nephrology/kidney-function/. Published August 2010. Accessed July 25, 2016.
Levey AS, Stevens LA, Coresh J. Conceptual model of CKD: applications and implications. Am J Kidney Dis. 2009;53(3 Suppl 3):S4-S16.
Marcellin P, Gane E, Flisiak R, et al. Long-term treatment with tenofovir disoproxil fumarate for chronic hepatitis B infection is safe and well tolerated and associated with durable virologic response with no detectable resistance: 8 year results from two phase 3 trials. AASLD. Boston. 2014. Slide deck (provided by Gilead).
Marieb EN, Hoehn K, eds. Human Anatomy and Physiology. 10th ed. Glenview, IL: Pearson Education Inc; 2016.
Martin LJ. At: MedlinePlus. BUN – blood test. https://www.nlm.nih.gov/medlineplus/ency/article/003474.htm. Updated April 30, 2015. Accessed June 20, 2016.
Mayo Clinic. Diseases and conditions: acute kidney failure. http://www.mayoclinic.org/diseases-conditions/kidney-failure/basics/definition/con-20024029?p=1. Updated June 5, 2015. Accessed July 25, 2016.
McMillan JI. At: Merck Manual Professional Edition. Chronic kidney disease. http://www.merckmanuals.com/professional/genitourinary-disorders/chronic-kidney-disease/chronic-kidney-disease. Updated October 2015. Accessed July 25, 2016.
Murphree DD, Thelen SM. Chronic kidney disease in primary care. J Am Board Fam Med. 2010;23:542-550.
National Kidney Disease Education Program. Urine albumin-to-creatinine ratio (UACR): in evaluating patients with diabetes for kidney disease. http://www.niddk.nih.gov/health-information/health-communication-programs/nkdep/a-z/quick-reference-uacr-gfr/Documents/quick-reference-uacr-gfr-508.pdf. Published March 2010. Accessed July 25, 2016.
National Kidney Foundation. Frequently asked questions about GFR estimates. https://www.kidney.org/sites/default/files/docs/12-10-4004_abe_faqs_aboutgfrrev1b_singleb.pdf. 2014. Accessed July 25, 2016.
National Kidney Foundation Kidney Disease Outcomes Quality Initiative. Clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. https://www.kidney.org/sites/default/files/docs/ckd_evaluation_classification_stratification.pdf. 2002. Accessed July 25, 2016.
Perazella MA. Renal vulnerability to drug toxicity. Clin J Am Soc Nephrol. 2009;4(7):1275-1283.
Pipili CL, Papatheodoridis GV, Cholongitas EC. Treatment of hepatitis B in patients with chronic kidney disease. Kidney Int. 2013;84(5):880-885.
Rahman M, Shad F, Smith MC. Acute kidney injury: a guide to diagnosis and management. Am Fam Physician. 2012;86(7):631-639.
Salgado JV, Neves FA, Bastos MG, et al. Monitoring renal function: measured and estimated glomerular filtration rates – a review. Braz J Med Biol Res. 2010;43:528-536.
Shah AP. At: Merck Manual Professional Version. Evaluation of the renal patient. http://www.merckmanuals.com/professional/genitourinary-disorders/approach-to-the-genitourinary-patient/evaluation-of-the-renal-patient. Updated May 2013. Accessed July 25, 2016.
Stedman's Online. Lippincott Williams & Wilkins. Copyright 2000-2016. http://www.stedmansonline.com/index. Accessed May 20-22, 2016.
Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function--measured and estimated glomerular filtration rate. N Engl J Med. 2006;354(23):2473-2483.
Taber's Online Dictionary. http://www.tabers.com/tabersonline. Accessed June 3, 2016.
Thompson EG. At: WebMd. Phosphate in blood. http://www.webmd.com/a-to-z-guides/phosphate-in-blood. Accessed August 19, 2016.
Tortora GJ, Derrickson B. Principles of Anatomy and Physiology. 14th ed. Hoboken, NJ: John Wiley & Sons Inc; 2014.
United States Renal Data System. Glossary. https://www.usrds.org/2015/appx/1_1_ADR_Glossary_15.pdf. Accessed June 3, 2016.
ATTESTATION
I attest that I have read all of the above materials.
ATTESTATION
You must view all interactive elements before you are marked complete.
